Our Services
Our Services
esqLABS develops and utilizes OPEN-SOURCE software to create physiologically-based Quantitative Systems Pharmacology (PB-QSP) platforms as value-generating, holistic Modeling & Simulation solutions to support our customers’ decision making process along the entire life cycle of pharmaceutical products from research through development and at the point of care.
PB-QSP Platform Development
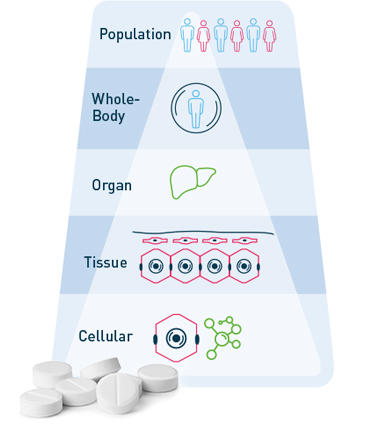
Today, in silico studies and trial simulations already complement experimental approaches in pharmaceutical R&D. However, project workflows and current software tools usually focus on isolated aspects of drug action, such as either pharmacokinetics at the organism scale or pharmacodynamic interaction (i.e. systems-biology) on the molecular level, even though biology is always multiscale by nature.
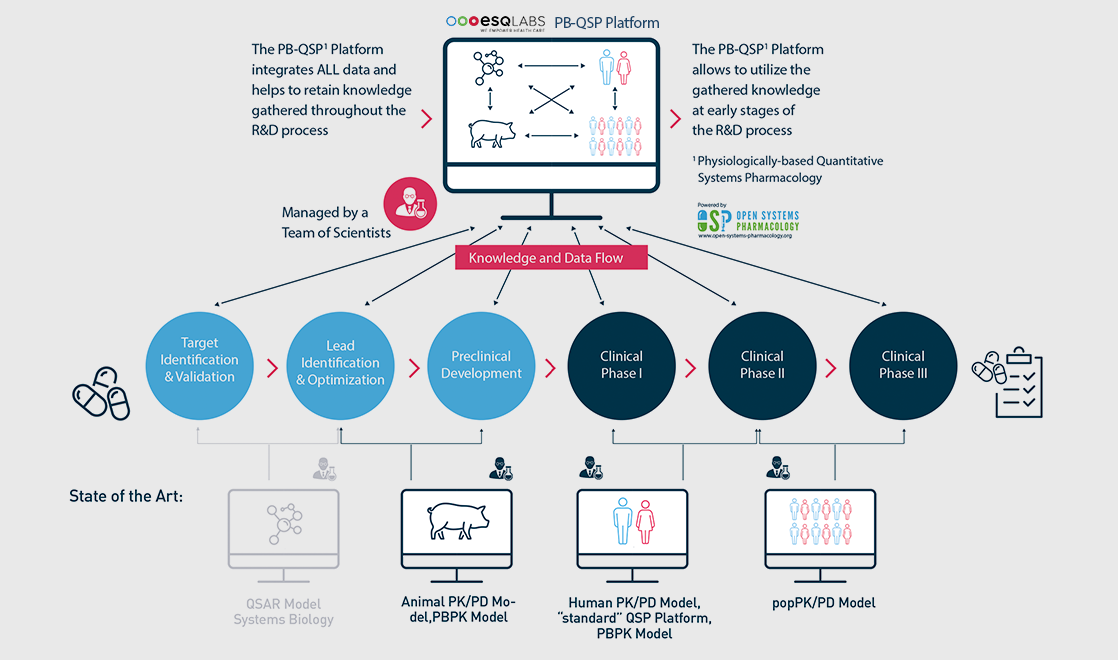
… within one single platform
The multi-scale physiologically-based (PB) modeling concept [1] in our PBPK models and PB-QSP platforms make it possible to integrate…
- multi-scale data from all biological scales (in-vitro, preclinical, individual and population-level clinical data)
- drug, disease, and physiology knowledge across multiple treatments within a therapeutic area
Through modeling & simulation, the multi-scale PB framework is then leveraged in pharma R&D and care for …
- consistent and quality-controlled build-up and transfer of actionable knowledge
- informed decision-support across all phases of drug research & development, and care

Our sophisticated model of glucose homeostasis …
… offers a powerful platform for research, drug development, and treatment personalization in the indication of Diabetes and metabolic diseases. The PB-QSP approach covers various scales from hormone-receptor interaction to population variability within different species. The backbone of our platform is open-source and under continuous development
Features
- Full PBPK representation of clinical biomarkers and treatments
- Glucose & HbA1c, multiple Insulins, Glucagon, GLP1, GIP, SGLT2 Inhibitors …
- … coupled with relevant PD mechanisms down to the insulin receptor level [2]
- Relevant preclinical species: rat, monkey & minipig [3]
- In-silico patient representations: healthy, T1DM, T2DM
- PK-modules: FcRn-binding, protein-Albumin binding, subcutaneous drug- and oral glucose- and meal absorption [2,4]
Informed & Validated
- With data from clinical trials, literature & partners [2,5,6]
- Tested in clinical trials within a closed-loop artificial pancreas system [5,6]
Efficiently leverage your preclinical experiments
- Physiologically-based framework to maximize learnings from animal experiments …
- … and translation to humans for a more efficient use of animal experiments
Assess in-licensing options and new drug derivatives
- Asses the effect of e.g. Fc-Fusion- or Albumin-binding approaches on compound PK & PD
- Assess potential benefit of drug combinations or multi-agonist entities
- Assess the optimal dose ratio for combination therapies
Clinical Trial Simulations
- Optimize trial design to ensure successful outcome (cohort selection, titration rules, measurement intervals and clinical endpoints)
- Assess treatment benefit in different populations (T1DM vs. T2DM)
- Benchmark precandidates vs. competitors for an optimized marketing strategy
- Optimize your medical device strategy by assessing effects of device properties on product & treatment efficacy
WITHIN A PROFESSIONAL, QUALITY-CONTROLLED PBPK/PD MODELING & SIMULATION FRAMEWORK [1]
List of Publicatons
- Eissing T, et al.: A computational systems biology software platform for multiscale modeling and simulation: Integrating whole-body physiology, disease biology, and molecular reaction networks. Front Physio 2011
- Schaller S, et al.: A generic integrated physiologically-based whole-body model of the glucose-insulin-glucagon regulatory system. CPT: PSP 2013
- Schaller S, Klabunde T: Towards Predictions of Clinical Trial Outcomes: Combining PBPK and QSP within a Translational Diabetes Disease Platform, PAGE Meeting 2018, Montreux
- Niederalt C, et al.: A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J Pharmacokinet. Pharmacodyn. 2018
- Schaller S, et al.: Robust MPC of blood glucose using generic whole-body physiology-based PK/PD model kernels. IEEE Transactions in Biomedical Engineering 2015.
- Schaller S: Automated Optimal Glycaemic Control using a Physiology-Based Pharmacokinetic/Pharmacodynamic Model. [PhD Thesis]: RWTH Aachen University 2014.
PBPK Consulting
In addition to PB-QSP platform development esqLABS also provides services & consulting for standard applications of PBPK models using the Open-Systems-Pharmacology Suite with PK-Sim® and MoBi®.
Services are:
- Preclinical/clinical base PBPK model development
- Special populations: PBPK-based pediatric investigations and organ impairment studies
- DDI Investigations
- Translational modeling for first-in-man dose predictions
- … and more. Contact us for details …
Trainings & Workshops
esqLABS provides on-demand E-Learning & individual trainings for customers on PBPK and PB-QSP modeling and simulation with PK-Sim® and MoBi®, taylored to your needs, starting at your level and bringing you to the edge in this field.
On a regular basis, we conduct workshops on how to best utilize PK-Sim® and MoBi® for your model-based drug development strategy. Topics span from applications for
- ADME and DMPK characterization: Preclinical/clinical base PBPK model development, PBPK-based pediatric extrapolation, Special Populations, DDI Investigations, translational modeling for first-in-man dose predictions …
- To utilizing the power of a physiologically-based framework for therapeutic-area specific questions related to disease physiology: combining PBPK and QSP approaches for quantitative analysis of mechanisms underlying a disease and its treatment.
A list of future events can be found in our News & Events section.
Get in touch now
Contact
ESQlabs GmbH | Am Sportplatz 7 | 26683 Saterland | Germany
Tel. +49 151 / 58559070 | info@esqLABS.com
This site does not use cookies to track your personal information
