New organ-on-chip pilot seeks to reduce animal testing in consumer health industry
A pilot project, supported by esqLABS, Dynamic42, Placenta Lab of Jena University Hospital, and Bayer’s Consumer Health Division

New organ-on-chip pilot
Animal testing has been a major ethical issue in the pharmaceutical industry, but now, a groundbreaking pilot project has just been launched! The project is set to combine cutting-edge “organ-on-chip” (OoC) technology with a digital twin to study the blood-placenta-barrier in pregnant women, a population that’s been underrepresented in clinical research due to the challenges involved.
This collaboration, between esqLABS, Dynamic42, Placenta Lab of Jena University Hospital, and Bayer’s Consumer Health Division, is expected to generate clinically relevant data, reduce costs, and enhance patient safety.
The project will utilize a microphysiological system that replicates the main human tissues involved in drug disposition, including the liver, intestine, and placenta. The system will simulate the distribution of compounds and translate the data into real-life human situations. This breakthrough technology could help to reduce animal testing while improving the outcomes of drug development.
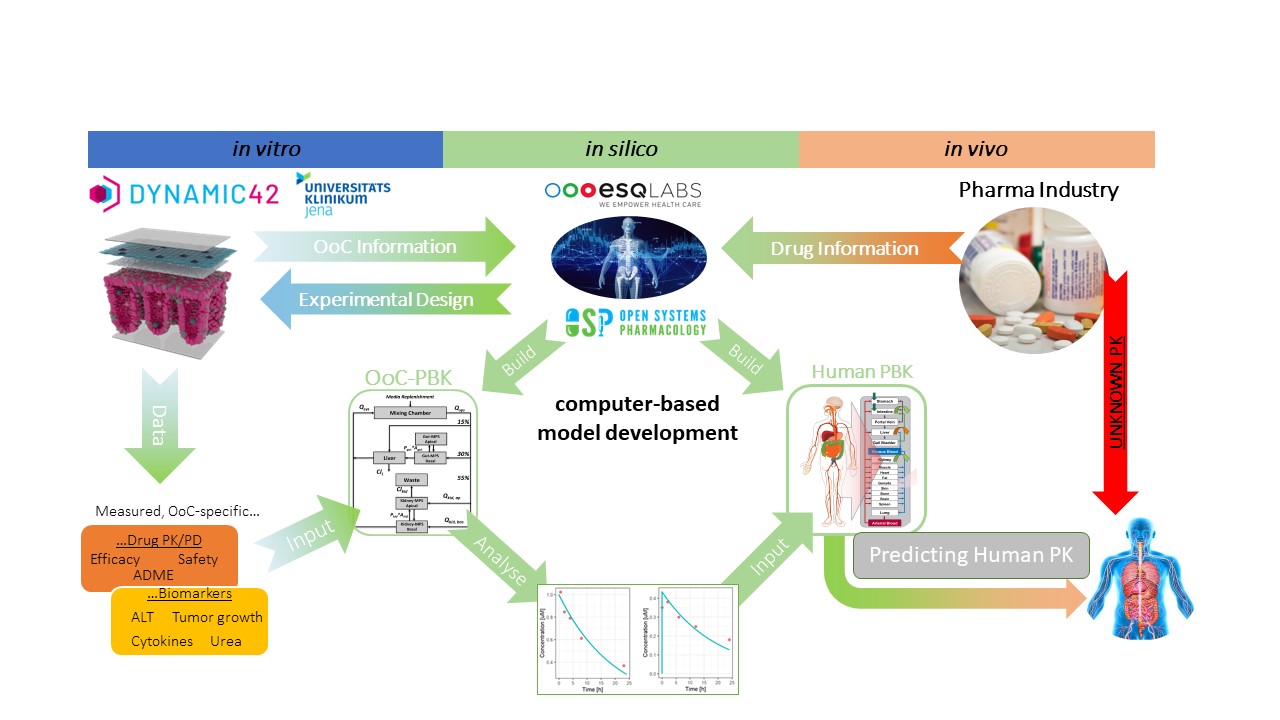
With the U.S. government approving new guidelines for pre-clinical drug testing that promote the use of innovative technologies and approaches to minimize animal testing, this project couldn’t have come at a better time. The joint project between esqLABS, Dynamic42, Placenta Lab of Jena University Hospital, and Bayer’s Consumer Health Division is set to change the game by finding novel ways to minimize animal testing and generate reliable and accurate data for product safety and efficacy.
Dr. Christian Maass, OoC-Research Lead at esqLABS, said, “We are thrilled to collaborate with scientists from Dynamic42, the Placenta Lab, and Bayer’s Consumer Health Division through this joint project to find novel ways to minimize animal testing and at the same time seek to generate more reliable and accurate data for product’s safety and efficacy.” Dr. Stephan Schaller, CEO of esqLABS, added, “This is an unprecedented endeavour, and we are very excited to be part of this journey. We all share the same vision.”
This pilot project is a milestone in the development of new mechanisms for studying the placenta barrier and its bi-directional selective transport in humans. The combined strengths of esqLABS, Dynamic42, and Bayer, along with the Placenta Lab’s experience in the field, will work synergistically to offer a path from bench to bedside.
Get in touch now
Contact
ESQlabs GmbH | Am Sportplatz 7 | 26683 Saterland | Germany
Tel. +49 151 / 58559070 | info@esqLABS.com
This site does not use cookies to track your personal information