esqLABS joins the 17 Mio € EU Project OnTOX
In May 2021, esqLABS joined the 17 Mio € EU Project OnTOX to provide a solution for advancing human risk assessment of chemicals without the use of animals

esqLABS receives public funding to develop computational NAMs
esqLABS is partnering with 17 institutions across the EU and the US to further develop computational approaches for next-generation risk assessment (NGRA) to provide a solution for advancing human risk assessment of chemicals without the use of animals.
esqLABS will lead WP4: “Kinetics” and WP2.3: “Quantitative Adverse Outcome Pathways”.
Within WP4, esqLABS will be responsible for the development of the QIVIVE framework, consisting of an integration of the generic PB(P)K modeling framework OSP Suite (PK-Sim and MoBi, www.open-systems-pharmacology.org) with cellular, and organ-level effect models, so-called Quantitative Adverse Outcome Pathways (qAOPs).
The unique modeling & simulation framework of the modeling software OSP Suite (PK-Sim and MoBi) will allow the integration of kinetics (PBK) and effects (qAOP) in an efficient manner to then integrate the model-based inference within the wider ecosystem of the AI-based and ontology-driven risk-assessment framework of ONTOX.
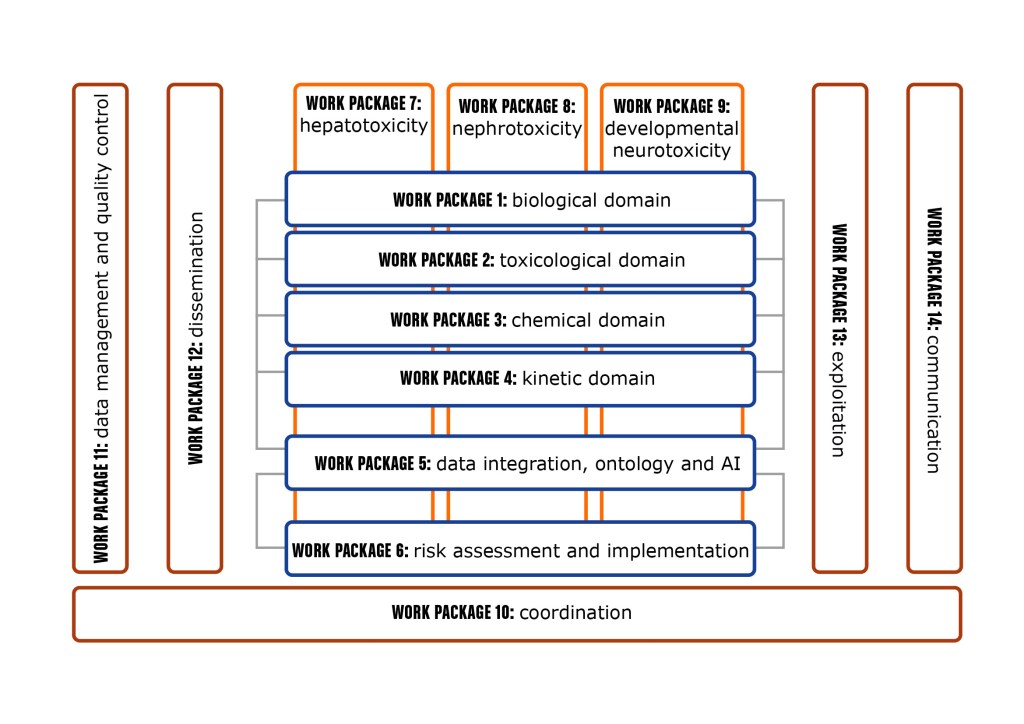
ONTOX will deliver a generic strategy to create innovative new approach methodologies (NAMs) in order to predict systemic repeated dose toxicity effects that, upon combination with tailored exposure assessment, will enable human risk assessment.
This strategy can be applied to any type of chemical and systemic repeated dose toxicity effect.
However, for proof-of-concept purposes, focus will be put on 6 specific NAMs addressing adversities in the liver (steatosis and cholestasis), kidneys (tubular necrosis and crystallopathy) and developing brain (neural tube closure and cognitive function defects) induced by a variety of chemicals, including from the pharmaceutical, cosmetics, food and biocide sectors.
The 6 NAMs will each consist of a computational system based on cutting-edge artificial intelligence (AI) and will be primarily fed by available biological / mechanistic, toxicological / epidemiological, physico-chemical and kinetic data. Data will be consecutively integrated in physiological maps, quantitative adverse outcome pathway networks and ontology frameworks. Data gaps, as identified by AI, will be filled by targeted state-of-the-art in vitro and in silico testing.
The 6 NAMs will be evaluated and applied in collaboration with industrial and regulatory stakeholders in order to maximise end-user acceptance and regulatory confidence.
This is anticipated to expedite implementation in risk assessment practice and to facilitate commercialisation.
ONTOX will have a deep and long-lasting impact at many levels, in particular by consolidating Europe’s world-leading position regarding the development, exploitation, regulation and application of animal-free methods for human risk assessment of chemicals.
Get in touch now
Contact
ESQlabs GmbH | Am Sportplatz 7 | 26683 Saterland | Germany
Tel. +49 151 / 58559070 | info@esqLABS.com
This site does not use cookies to track your personal information

 Belgium
Belgium